
HL Paper 1
Given the enthalpy change for the reaction below:
\[\begin{array}{*{20}{l}} {{\text{2}}{{\text{H}}_{\text{2}}}{\text{(g)}} + {{\text{O}}_{\text{2}}}{\text{(g)}} \to {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}}}&{\Delta {H^\Theta } = - 572{\text{ kJ}}} \end{array}\]
which statement is correct?
A. The standard enthalpy change of combustion of \({{\text{H}}_2}{\text{(g)}}\) is \( - 286{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
B. The standard enthalpy change of combustion of \({{\text{H}}_2}{\text{(g)}}\) is \( + 286{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
C. The standard enthalpy change of formation of \({{\text{H}}_2}{\text{O(l)}}\) is \( - 572{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
D. The standard enthalpy change of formation of \({{\text{H}}_2}{\text{O(l)}}\) is \( + 572{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
Markscheme
A
Examiners report
Consider the two reactions involving iron and oxygen.
\[\begin{array}{*{20}{l}} {{\text{2Fe(s)}} + {{\text{O}}_2}{\text{(g)}} \to {\text{2FeO(s)}}}&{\Delta {H^\Theta } = - 544{\text{ kJ}}} \\ {{\text{4Fe(s)}} + {\text{3}}{{\text{O}}_2}{\text{(g)}} \to {\text{2F}}{{\text{e}}_2}{{\text{O}}_3}{\text{(s)}}}&{\Delta {H^\Theta } = - 1648{\text{ kJ}}} \end{array}\]
What is the enthalpy change, in kJ, for the reaction below?
\[{\text{4FeO(s)}} + {{\text{O}}_2}{\text{(g)}} \to {\text{2F}}{{\text{e}}_2}{{\text{O}}_3}{\text{(s)}}\]
A. \( - 1648 - 2( - 544)\)
B. \( - 544 - ( - 1648)\)
C. \( - 1648 - 544\)
D. \( - 1648 - 2(544)\)
Markscheme
A
Examiners report
One respondent stated that it would have been better to represent the four choices A-D as numerical values. However, candidates do not have access to a calculator in P1 and therefore simply had to use Hess’s law without working out the final answer. 67.83% of candidates got the correct answer.
Enthalpy changes of reaction are provided for the following reactions.
\[\begin{array}{*{20}{l}} {{\text{2C(s)}} + {\text{2}}{{\text{H}}_2}{\text{(g)}} \to {{\text{C}}_2}{{\text{H}}_4}{\text{(g)}}}&{\Delta {H^\Theta } = + {\text{52 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \\ {{\text{2C(s)}} + {\text{3}}{{\text{H}}_2}{\text{(g)}} \to {{\text{C}}_2}{{\text{H}}_6}{\text{(g)}}}&{\Delta {H^\Theta } = - {\text{85 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \end{array}\]
What is the enthalpy change, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for the reaction between ethene and hydrogen?
\[{{\text{C}}_2}{{\text{H}}_4}{\text{(g)}} + {{\text{H}}_2}{\text{(g)}} \to {{\text{C}}_2}{{\text{H}}_6}{\text{(g)}}\]
A. –137
B. –33
C. +33
D. +137
Markscheme
A
Examiners report
The enthalpy change for the dissolution of NH4NO3 is +26 kJ mol–1 at 25 °C. Which statement about this reaction is correct?
A. The reaction is exothermic and the solubility decreases at higher temperature.
B. The reaction is exothermic and the solubility increases at higher temperature.
C. The reaction is endothermic and the solubility decreases at higher temperature.
D. The reaction is endothermic and the solubility increases at higher temperature.
Markscheme
D
Examiners report
Which equation represents the standard enthalpy of formation of liquid methanol?
A. \({\text{C(g) + 2}}{{\text{H}}_{\text{2}}}{\text{(g) + }}\frac{1}{2}{{\text{O}}_{\text{2}}}{\text{(g)}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{OH(l)}}\)
B. \({\text{C(g) + 4H(g) + O(g)}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{OH(l)}}\)
C. \({\text{C(s) + 4H(g) + O(g)}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{OH(l)}}\)
D. \({\text{C(s) + 2}}{{\text{H}}_{\text{2}}}{\text{(g) + }}\frac{1}{2}{{\text{O}}_{\text{2}}}{\text{(g)}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{OH(l)}}\)
Markscheme
D
Examiners report
This was a fair question with half the students answering it correctly. It was a definition question on the standard enthalpy of formation of liquid methanol but almost 40% of the candidates selected choice A where carbon is given in the gaseous state, not its solid under standard conditions.
Which reaction has an enthalpy change equal to the standard enthalpy change of combustion?
A. \({{\text{C}}_3}{{\text{H}}_8}{\text{(g)}} + {\text{5}}{{\text{O}}_2}{\text{(g)}} \to {\text{3C}}{{\text{O}}_2}{\text{(g)}} + {\text{4}}{{\text{H}}_2}{\text{O(g)}}\)
B. \({{\text{C}}_3}{{\text{H}}_8}{\text{(g)}} + {\text{5}}{{\text{O}}_2}{\text{(g)}} \to {\text{3C}}{{\text{O}}_2}{\text{(g)}} + {\text{4}}{{\text{H}}_2}{\text{O(l)}}\)
C. \({\text{2}}{{\text{C}}_4}{{\text{H}}_{10}}{\text{(g)}} + {\text{13}}{{\text{O}}_2}{\text{(g)}} \to {\text{8C}}{{\text{O}}_2}{\text{(g)}} + {\text{10}}{{\text{H}}_2}{\text{O(l)}}\)
D. \({{\text{C}}_5}{{\text{H}}_{12}}{\text{(g)}} + {\text{8}}{{\text{O}}_2}{\text{(g)}} \to {\text{5C}}{{\text{O}}_2}{\text{(g)}} + {\text{6}}{{\text{H}}_2}{\text{O(g)}}\)
Markscheme
B
Examiners report
Which process is endothermic?
A. \({\text{2}}{{\text{C}}_4}{{\text{H}}_{10}}{\text{(g)}} + {\text{13}}{{\text{O}}_2}{\text{(g)}} \to {\text{8C}}{{\text{O}}_2}{\text{(g)}} + {\text{10}}{{\text{H}}_2}{\text{O(g)}}\)
B. \({\text{Na(g)}} \to {\text{N}}{{\text{a}}^ + }{\text{(g)}} + {{\text{e}}^ - }\)
C. \({{\text{H}}_2}{\text{S}}{{\text{O}}_4}{\text{(aq)}} + {\text{2KOH(aq)}} \to {{\text{K}}_2}{\text{S}}{{\text{O}}_4}{\text{(aq)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}}\)
D. \({\text{N}}{{\text{H}}_3}{\text{(g)}} \to {\text{N}}{{\text{H}}_3}{\text{(l)}}\)
Markscheme
B
Examiners report
Which equation represents the bond enthalpy for the H–Br bond in hydrogen bromide?
A. \({\text{HBr(g)}} \to {\text{H(g)}} + {\text{Br(g)}}\)
B. \({\text{HBr(g)}} \to {\text{H(g)}} + {\text{Br(l)}}\)
C. \({\text{HBr(g)}} \to {\text{H(g)}} + \frac{1}{2}{\text{B}}{{\text{r}}_2}({\text{l)}}\)
D. \({\text{HBr(g)}} \to {\text{H(g)}} + \frac{1}{2}{\text{B}}{{\text{r}}_2}({\text{g)}}\)
Markscheme
A
Examiners report
The same amount of heat energy is added to 1.00 g of each substance.
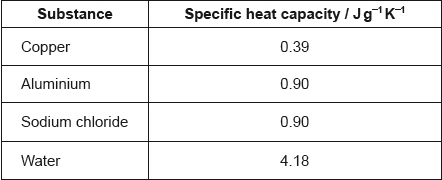
Which statement is correct if all the substances are at the same temperature before the heat energy is added?
A. Copper will reach the highest temperature.
B. Water will reach the highest temperature.
C. All four substances will reach the same temperature.
D. Aluminium will reach a higher temperature than sodium chloride.
Markscheme
A
Examiners report
1.0 g of sodium hydroxide, NaOH, was added to 99.0 g of water. The temperature of the solution increased from 18.0 °C to 20.5 °C. The specific heat capacity of the solution is \({\text{4.18 J}}\,{{\text{g}}^{ - 1}}{{\text{K}}^{ - 1}}\). Which expression gives the heat evolved in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)?
A. \(\frac{{2{\text{.}}5 \times 100.0 \times 4.18 \times 1000}}{{40{\text{.}}0}}\)
B. \(\frac{{2{\text{.}}5 \times 100.0 \times 4.18}}{{1000 \times 40{\text{.}}0}}\)
C. \(\frac{{2{\text{.}}5 \times 100.0 \times 4.18 \times 40{\text{.}}0}}{{1000}}\)
D. \(\frac{{2{\text{.}}5 \times 1{\text{.}}0 \times 4{\text{.}}18 \times 40{\text{.}}0}}{{1000}}\)
Markscheme
C
Examiners report
One respondent felt that this question was unnecessarily complicated. This was discussed at Grade Award and although there is some validity to this statement, it was considered that the question was fair but was considered one of the harder questions on the paper. With an associated difficulty index of 34.69%, the question was in fact the fifth hardest question overall.
Consider the equations below.
\[\begin{array}{*{20}{l}} {{\text{C}}{{\text{H}}_{\text{4}}}{\text{(g)}} + {{\text{O}}_{\text{2}}}{\text{(g)}} \to {\text{HCHO(l)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}}}&{\Delta {H^\Theta } = x} \\ {{\text{HCHO(l)}} + \frac{1}{2}{{\text{O}}_2}{\text{(g)}} \to {\text{HCOOH(l)}}}&{\Delta {H^\Theta } = y} \\ {2{\text{HCOOH(l)}} + \frac{1}{2}{{\text{O}}_2}{\text{(g)}} \to {{{\text{(COOH)}}}_2}({\text{s)}} + {{\text{H}}_2}{\text{O(l)}}}&{\Delta {H^\Theta } = z} \end{array}\]
What is the enthalpy change of the reaction below?
\[2{\text{C}}{{\text{H}}_4}{\text{(g)}} + {\text{3}}\frac{1}{2}{{\text{O}}_2}{\text{(g)}} \to {{\text{(COOH)}}_2}{\text{(s)}} + 3{{\text{H}}_2}{\text{O(l)}}\]
A. \(x + y + z\)
B. \(2x + y + z\)
C. \(2x + 2y + z\)
D. \(2x + 2y + 2z\)
Markscheme
C
Examiners report
On one of the G2’s it was stated that this question was challenging as candidates are not used to dealing with three equations when the enthalpy change asked for specifically involves only two organic compounds. This question is based on AS 5.3.1 which states explicitly that candidates should be able to determine the enthalpy change of a reaction that is the sum of two or three reactions with known enthalpy changes. In this question three reactions were given with corresponding enthalpy change values of x, y and z. Hence, by fairly straight-forward manipulation of the reactions, the final enthalpy change of the given reaction could be determined as \(C = 2x + 2y + z\). The question was answered correctly by 84% of candidates and in fact was found to be the seventh easiest question on the paper with a corresponding discrimination index of 0.32.
Which processes are exothermic?
I. \({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}{\text{(g)}} + {\text{5}}{{\text{O}}_2}{\text{(g)}} \to {\text{3C}}{{\text{O}}_2}{\text{(g)}} + {\text{4}}{{\text{H}}_2}{\text{O(g)}}\)
II. \({\text{C}}{{\text{l}}_2}{\text{(g)}} \to {\text{2Cl(g)}}\)
III. \({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{COOH(aq)}} + {\text{NaOH(aq)}} \to {\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{COONa(aq)}} + {{\text{H}}_2}{\text{O(l)}}\)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
B
Examiners report
Which equation represents the standard enthalpy change of formation, \(\Delta H_f^\Theta \), of tetrachloromethane?
A. \({\text{C(g)}} + {\text{4Cl(g)}} \to {\text{CC}}{{\text{l}}_{\text{4}}}{\text{(g)}}\)
B. \({\text{C(s)}} + {\text{4Cl(g)}} \to {\text{CC}}{{\text{l}}_{\text{4}}}{\text{(l)}}\)
C. \({\text{C(g)}} + {\text{2C}}{{\text{l}}_{\text{2}}}{\text{(g)}} \to {\text{CC}}{{\text{l}}_{\text{4}}}{\text{(g)}}\)
D. \({\text{C(s)}} + {\text{2C}}{{\text{l}}_{\text{2}}}{\text{(g)}} \to {\text{CC}}{{\text{l}}_{\text{4}}}{\text{(l)}}\)
Markscheme
D
Examiners report
It is accepted that candidates should not have come across tetrachloromethane, \({\text{CC}}{{\text{l}}_{\text{4}}}\), it having been banned for many years. They were not expected to know that it is a liquid at room temperature and the question can be answered correctly without this knowledge. A standard enthalpy change of formation must start from the elements so only C and D are possible. Carbon is not a gas under standard conditions so answer C is excluded. Nearly 47% gave the correct answer.
Consider the following two equations.
\({\text{2Ca(s)}} + {{\text{O}}_2}{\text{(g)}} \to {\text{2CaO(s)}}\) \(\Delta {H^\Theta } = + x{\text{ kJ}}\)
\({\text{Ca(s)}} + {\text{0.5}}{{\text{O}}_2}{\text{(g)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}} \to {\text{CaC}}{{\text{O}}_3}{\text{(s)}}\) \(\Delta {H^\Theta } = + y{\text{ kJ}}\)
What is \(\Delta {H^\Theta }\), in kJ, for the following reaction?
\({\text{CaO(s)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}} \to {\text{CaC}}{{\text{O}}_3}{\text{(s)}}\)
A. \(y - 0.5x\)
B. \(y - x\)
C. \(0.5 - y\)
D. \(x - y\)
Markscheme
A
Examiners report
This was a common question with standard level where there was concern about the use of algebraic notation rather than actual numerical data. Algebraic notation has been used since November 2010 so candidates should be familiar with this type of question.
While one comment in HL agreed with this sentiment, the other said it was “good to use pronumerals”. In the event, it was the fifth easiest question; nearly 91% of candidates gave the correct answer and less than 6% gave B.
Which ionic compound has the most endothermic lattice enthalpy?
A. Sodium chloride
B. Sodium oxide
C. Magnesium chloride
D. Magnesium oxide
Markscheme
D
Examiners report
This question was thought to be “ambiguous as lattice energy can be defined as an exothermic or endothermic process”. Students should be familiar with the idea of lattice energy as an endothermic process as that is how it is described in the IB Chemistry Data Booklet. A similar question was set in May 2011, TZ2.
This was the fourth hardest question, being scored correctly by 55.81%. The other answers were fairly evenly spread between A, C and B (in that order).
The combustion of glucose is exothermic and occurs according to the following equation:
C6H12O6 (s) + 6O2 (g) → 6CO2 (g) + 6H2O (g)
Which is correct for this reaction?
Markscheme
A
Examiners report
B. 2 × (−394) + (−572) − (−2602)
C. 2 × (−394) + \(\frac{1}{2}\) (−572) + \(\frac{1}{2}\) (−2602)
D. 2 × (−394) + (−572) + (−2602)
Markscheme
A